HeartWare Buys NJ Firm For $30M
 Courtesy of HeartWare International
HeartWare International of Framingham has bought CircuLite of New Jersey in a deal that could wind up being worth $350 million.
Courtesy of HeartWare International
HeartWare International of Framingham has bought CircuLite of New Jersey in a deal that could wind up being worth $350 million.
HeartWare International of Framingham has bought a New Jersey-based medical device manufacturer in a deal that could wind up being worth $350 million.
HeartWare said it purchased Teaneck-based CircuLite Inc., which helps treats patients with early-stage heart failure, for $18 million in common stock and $12 million in cash to settle CircuLite’s outstanding debt. The remaining $320 million is contingent upon satisfying regulatory and commercial milestones, the companies said.
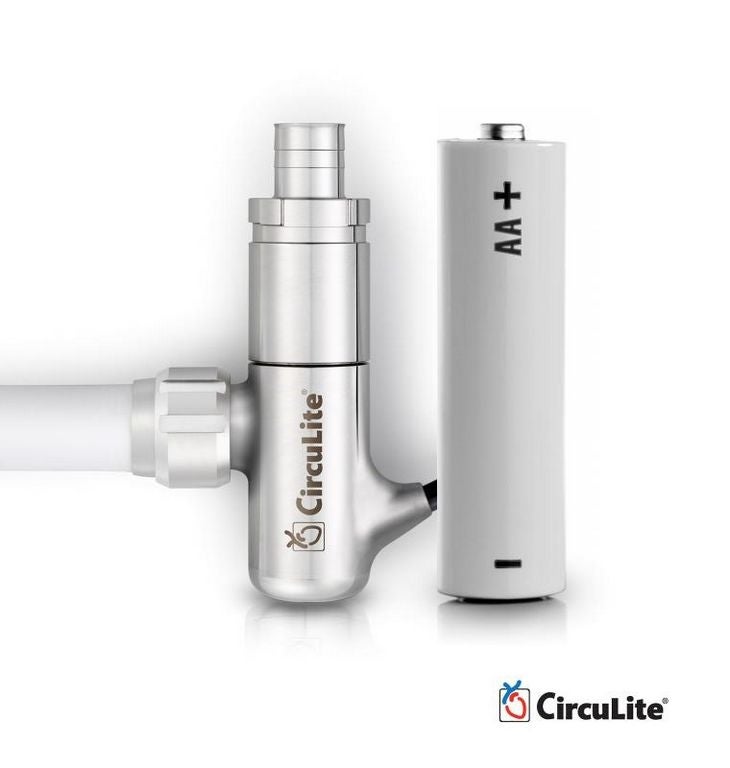
HeartWare said it acquired CircuLite due to the success of its devices, notably the Synergy heart pump, which received European Union (EU) certification in 2012.
Synergy is being upgraded after finding last summer that a tube on the pump could fracture, and it will receive additional investment to expand the release as a result of the acquisition.
“The partial-support system developed by CircuLite represents the industry’s most intriguing platform for the treatment of patients with earlier stage heart failure,” HeartWare President and CEO Doug Godshall said in a statement. “CircuLite has pioneered the partial-assist approach and demonstrated that this technique can significantly enhance the quality of life for this group of patients.”
“We are confident that HeartWare’s technical, regulatory, sales and marketing capabilities will have a profound impact on enabling the Synergy system to reach its full potential,” said Dr. Daniel Burkhoff, CircuLite’s chief medical officer.
The other potential payments include:
- $20 million once the Synergy is back on the market in EU countries;
- $75 million once the EU approves a product for heart patients who can respond to standard treatment (interventional system);
- $50 million once their system wins approval from the U.S. Food and Drug Administration;
- $15 million once annual sales of Synergy reach $30 million;
- $85 million once annual sales of Synergy reach $250 million; and
- $75 million for further sales of Synergy or the interventional system
Read more
HeartWare Stock To Delist In Australia













0 Comments