BSX expanding defibrillator line into Asia
 COURTESY
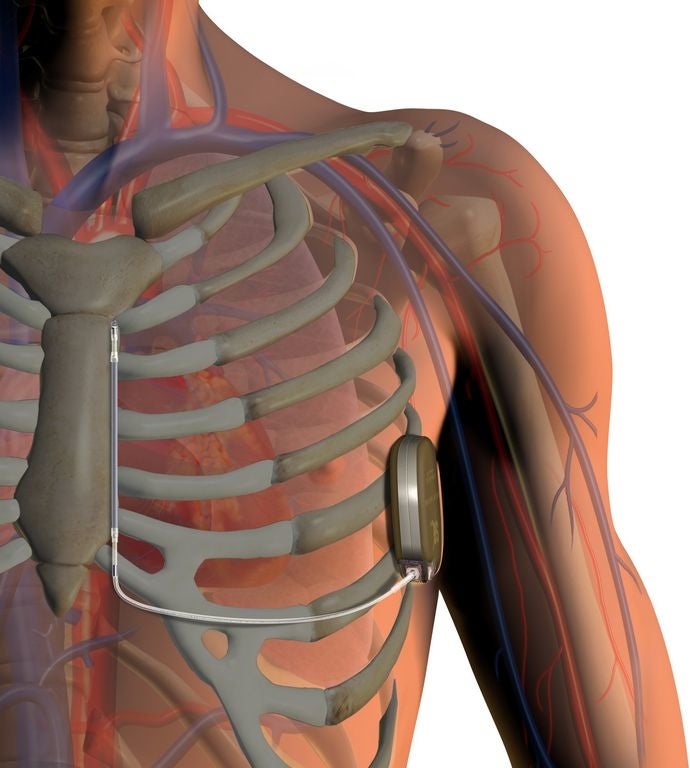
Boston Scientific's newly approved defibrillator is unique because doctors don't need to attach electrodes to the heart.
COURTESY
Boston Scientific's newly approved defibrillator is unique because doctors don't need to attach electrodes to the heart.
Natick-based Boston Scientific has expanded the rollout of its implantable defibrillator into parts of Asia.
The first Asian implant of the subcutaneous implantable cardioverter defibrillator, or S-ICD system, was performed March 31 in Hong Kong. BSX said the device doesn’t have regulatory approval in China, Japan or South Korea and therefore isn’t available for sale in those countries.
Approximately two million people in the Asia-Pacific region are at risk of sudden cardiac arrest, according to BSX, which is caused mostly by rapid or chaotic activity of the heart.
“I envision the S-ICD System to be the first-choice solution for eligible patients because it provides a less invasive therapy,” said Hung Fat Tse, who performed the continent’s first S-ICD implant.
The S-ICD is the only device maker that rests below the skin and doesn’t touch the heart or blood vessels as “transvenous” ICDs do, BSX said.
The system was approved for use in Europe in 2009 and was implanted in more than 1,400 patients in its first three years, according to BSX. It has been gradually rolled out across the United States after receiving regulatory approval in late 2012.
BSX added the S-ICD to its product line with the acquisition of California-based Cameron Health Inc. in June 2012 for $150 million.
BSX paid another $150 million to Cameron after the S-ICD won approval from the U.S. Food and Drug Administration (FDA), and is on the hook for an additional $1.05 billion if specific revenue-based goals for the S-ICD are reached over the next four years.












0 Comments