Marlborough medical device manufacturer Boston Scientific Corp. announced on Thursday it is launching a clinical trial of its mCRM Modular Therapy System.
The mCRM Modular Therapy System consists of two cardiac rhythm management devices working together to coordinate therapy.
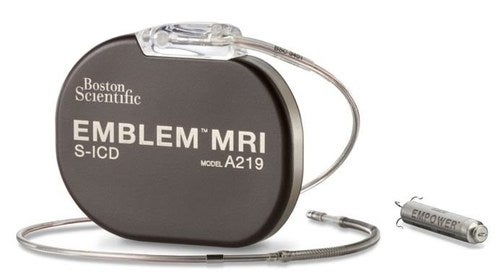
The two parts of the device are the EMBLEM MRI Subcutaneous Implantable Defibrillator System and the EMPOWER Modular Pacing System, which is designed to be the first leadless pacemaker capable of delivering both bradycardia pacing support and antitachycardia pacing, according to the press release.
“The components of the system are designed to work in concert with each other, regardless of when implanted, giving physicians the ability to provide personalized patient care today while keeping options open in the future,” said Dr. Kenneth Stein, senior vice president and chief medical officer of Rhythm Management and Global Health Policy at Boston Scientific.
The clinical trial will take place at 50 medical centers throughout the United States, Canada, and Europe, and will include up to 300 patients.