ReWalk Robotics hopes to eventually reach profitability after the U.S. Food & Drug Administration last week approved the ReStore, an exoskeleton system designed to help stroke patients rehab and eventually walk again. The company also has its ReWalk device, meant to help patients with spinal cord injuries. In an interview, CEO Larry Jasinski spoke about the company’s next steps and path to profitability.
How exciting was last week for the company?
For us as a company, we’ve been working on this for four years. At Harvard University, they were working on this for four years before that. There have been a lot of patients and doctors. It’s a change for the company obviously in that it sets up the strategy we had of having multiple product lines to get to profitability. For wearable robotics, there’s a lot of noise about it because this is the first product to get FDA clearance. It has an impact on the entire industry.
Four years is a pretty quick turnaround for a medical device.
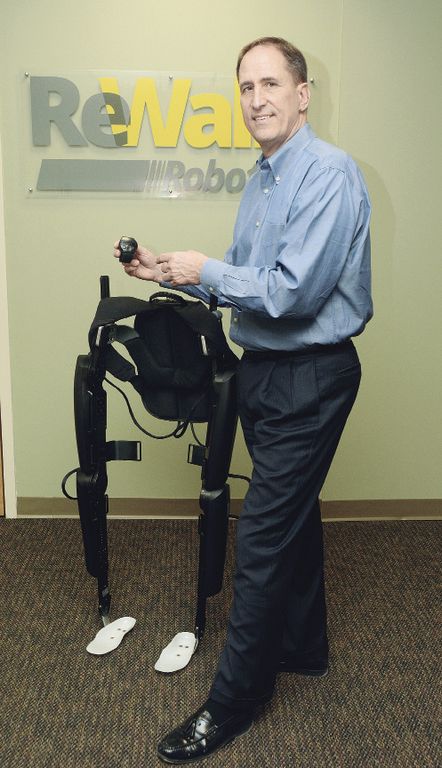
It was. We acquired the rights to it in 2015 and had it for about four years as an active program. Harvard worked on it for several years before that.
Harvard came to us because that’s something we do very well. We do very well in getting regulatory approvals and clinical studies done.
What does it feel like to finally clear what is probably your biggest hurdle?
It is a big one. There’s still all the fundamentals from creation to building a product that works to clinical trials to regulatory and production. So we’ve gone through all the normal phases, and now we’re in the commercialization phase.
We already have a leasing program set up through a vendor allowing clinics to acquire the systems at an economical price for them and their business. Most American groups not buying the system outright can lease it for $1,140 per month.
What is the company doing to prepare for U.S. sales?
We started training employees a few weeks ago in anticipation of FDA approval. We’re going to place systems at a reasonable number of centers to start, but this should really be a growth vehicle for us, particularly next year and the year after. If you’re trying to drive a company with the mindset of breaking even or profitability – we have the ReWalk with modest growth, and are expecting rapid growth with the ReStore – that’s the path to a sustainable company.
When will the first systems be sold?
We got FDA clearance last week, so any day now.
How many rehab centers are in line for a ReStore to start?
In the U.S., I describe this as a Main Street product. There’s about 1,00 Tier 1 centers that will be candidates initially. Behind that, there’s 10s of thousands of smaller centers. There will be typically multiple devices per center at this price point.
With both products approved in the U.S., will ReWalk begin really exploring other applications?
We’re looking at a stroke system that works at the hip. We also have patients being treated for multiple sclerosis and Parkinson’s disease.
This interview was conducted and edited for length and clarity by WBJ Staff Writer Zachary Comeau.

