Boston Scientific issues recall for failing surgical instrument
 Courtesy Boston Scientific
Courtesy Boston Scientific
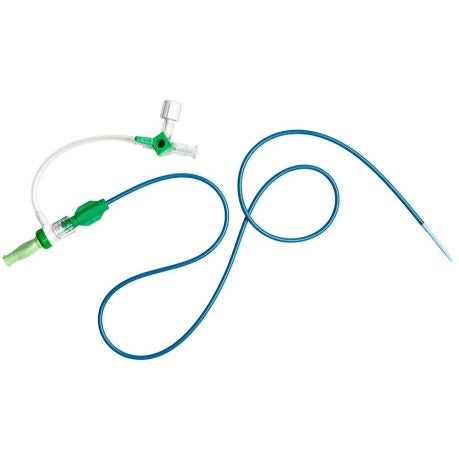
Marlborough-based Boston Scientific has issued a global recall on its Chariot Guiding Sheath following 14 complaints of failure, including while the device was inside patients, which could lead to obstruction of blood flow causing stroke or kidney damage.
This voluntary recall has been classified as a Class-1 recall by the U.S. Food and Drug Administration. Guiding sheaths are used to enter into the vascular system for intervention or diagnosis.
There have been 21 reports of failure of the Chariot sheath on the FDA’s website. These failures include pieces of the sheath separating, sometimes while the device was within a patient. There have been no reports of permanent injuries or patient deaths, according to Boston Scientific.
The company contacted healthcare facilities on Nov. 19, advising them to immediately discontinue the affected devices and return them to the company.













0 Comments