Marlborough co. gets FDA approval for laser heart treatment
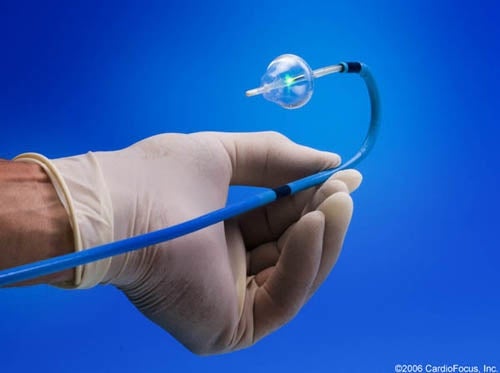 Courtesy CardioFocus
The CardioFocus treatment uses a steerable sheath.
Courtesy CardioFocus
The CardioFocus treatment uses a steerable sheath.
Marlborough’s Boston Biomedical Associates (BBA) has achieved premarket approval from the FDA for Boston-based CardioFocus’ laser therapy that treats irregular heartbeats caused by atrial fibrillation.
BBA assisted with the approval process for CardioFocus’ HeartLight Endoscopic Ablation System that removes or eradicates tissue using a laser in order to return the heart to its regular beat. This system, according to CardioFocus, allows for a minimally invasive two to three hour surgery using an endoscope.
The premarket approval, which allows the company to market the treatment, came after a 350 person study across 21 U.S. medical centers. With the granting of the approval, CardioFocus becomes one of few manufacturers possessing a specific indication for catheter ablation therapy of paroxysmal atrial fibrillation.
Boston Biomedical Associates is a full-service clinical research organization that works with companies throughout the entire medical product lifecycle to help them bring new technologies to market.









0 Comments