Less than four months after completing its acquisition of Cameron Health Inc. for $150 million, Natick-based Boston Scientific (BSX) has won regulatory approval for a United States rollout of a unique defibrillator developed by Cameron.
The subcutaneous implantable cardioverter defibrillator, or S-ICD System, is for patients who are at risk of sudden cardiac arrest, which is mostly caused by rapid or chaotic activity of the heart. Approximately 850,000 people in the United States are at risk of the condition, according to BSX.
The device is the only defibrillator of its kind, and BSX said it expects it to remain that way for several years.
A Unique Product
“With the addition of the S-ICD System, we believe Boston Scientific has a compelling and highly differentiated portfolio that will help fuel our growth strategy,” said Hank Kucheman, the company’s CEO.
Kucheman said BSX is the only device maker so far to offer an ICD that rests just below the skin.
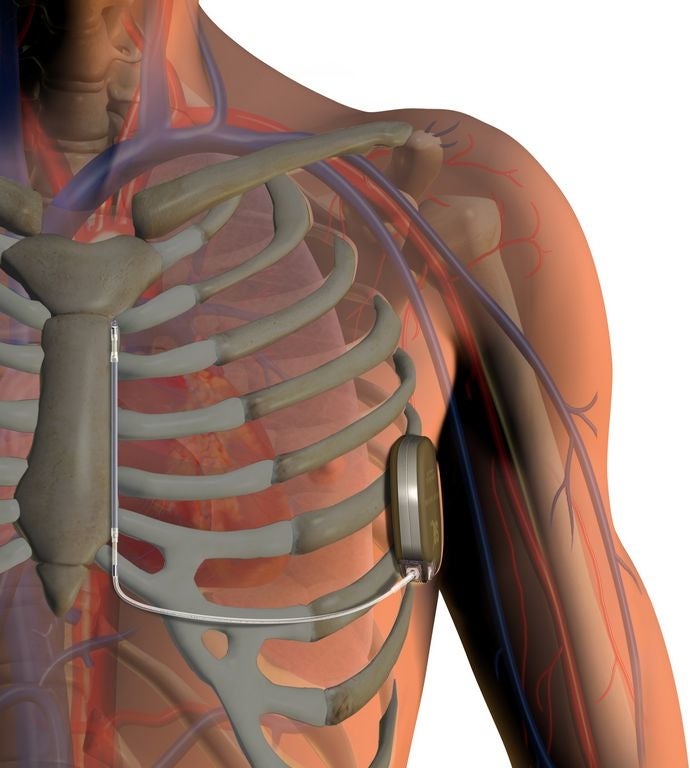
The S-ICD is unique because it doesn’t touch the heart or blood vessels, as “transvenous” ICDs do.
The system has a pulse generator that powers the system, monitors heart activity and delivers a therapeutic shock, if needed, and an electrode, which allows it to sense heart rhythm and deliver the shocks.
Both parts are implanted just under the skin, at the side of the chest and besides the breastbone, respectively. Unlike its predecessors, the S-ICD does not require that thin, insulated wires be placed in the heart or blood vessels. And that means the X-ray procedure required to insert those wires is also unnecessary.
“The S-ICD System provides an alternative for treating patients with life-threatening heart arrhythmias for whom the routine ICD placement procedure is not ideal,” said Christy Foreman, director of the Office of Device Evaluation at FDA’s Center for Devices and Radiological Health. “Some patients with anatomy that makes it challenging to place one of the implantable defibrillators currently on the market may especially benefit from this device.”
Revenue Sharing
The former owners of Cameron Health could reap the benefits of a U.S. launch of the device, even though they sold the company.
In addition to the $150 million it already paid for Cameron, BSX could pay up to an addition $150 million now that the device has received approval, it disclosed in its announcement of the sale earlier this year.
BSX also said it could pay up to just over $1.05 billion in revenue-sharing payments to Cameron if certain revenue-based criteria are met for the S-ICD System over a six-year period.
BSX plans to launch the product in phases in the United States. The system is already in use in Europe. It received the equivalent approval there, known as CE Mark, in 2009, and has been implanted in more than 1,400 patients.
It is approved only for patients who do not require a pacemaker or pacing therapy.
The FDA is requiring Cameron to conduct a post-market study to assess the long-term safety and performance of the defibrillator and to assess differences in effectiveness across genders.
Read more