ReWalk exoskeleton approved for surgeon; deemed ‘medically necessary’
 WBJ FILE PHOTO
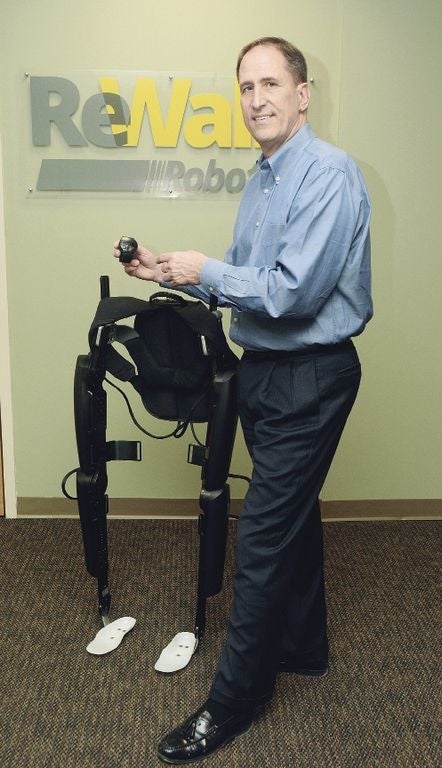
ReWalk Robotics CEO Larry Jasinski displays a ReWalk system at
the company's U.S. headquarters in Marlborough in February.
WBJ FILE PHOTO
ReWalk Robotics CEO Larry Jasinski displays a ReWalk system at
the company's U.S. headquarters in Marlborough in February.
ReWalk Robotics has passed another milestone as its personal exoskeleton system has been approved by a commercial health plan in the United States for use by a surgeon who will now be able to stand at work.
The exoskeleton from the company, which has U.S. headquarters in Marlborough, will allow the surgeon who suffered a spinal cord injury and currently uses a manual custom wheelchair 11 hours a day at work to stand while at the hospital and at home.
The health plan provider's coverage approval follows the ruling of an external independent review organization, which overturned the company's initial denial of coverage, according to ReWalk. The independent medical review organization determined that the ReWalk system is not an experimental or investigational technology, citing "sufficient evidence found in current peer-reviewed medical literature to support the use of the ReWalk device in patients with spinal cord injury."
"The ruling by the independent medical organization marks an important moment for exoskeletons being accepted as protocol technology for those with spinal cord injury," Larry Jasinski, ReWalk CEO, said in a statement. "Health benefit providers have historically been hesitant to acknowledge the clinical benefits in their case assessments. This ruling, and subsequent coverage and reimbursement will help ReWalk in our efforts to facilitate greater patient access to the device."
This commercial health plan approval follows the recent news that the U.S. Department of Veterans Affairs issued a national policy for the evaluation, training and procurement of ReWalk Personal exoskeleton systems for all qualifying veterans across the United States. The VA is also currently undertaking a 160-person study of the system. ReWalk is currently the only FDA cleared exoskeleton technology for individuals with spinal cord injury.










0 Comments